Product Categories
- Related Products (0)
- Main products (5)
- Minoxidil (1)
- Finasteride (1)
- Dutasteride (1)
- RU58841 (1)
- Way316606 (1)
- New Research (4)
- CB-03-01 (1)
- KX-826 (1)
- SM04554 (1)
- Pyrrolidinyl Diaminopyrimidine (1)
Today, we talk about a new hair loss treatment product called SM04554.SM04554 is called the ultimate solution to baldness.
In baldness, there is a process called miniaturization, in which the hair becomes thinner and shorter until it disappears completely, at which point, when the skin on the scalp becomes smooth and shiny, it manifests as baldness, and it is often believed that any medical treatment is too late for people who are already balding.
Until SM0455, balding patients were able to regrow their hair.
SM04554 is an experimental drug that has completed clinical trials. It was developed by Samumed.
Known for its innovative work on the Wnt pathway, Samumed has successfully developed small molecule drugs that may address a variety of degenerative diseases, and SM04554 has also been described as a “novel small molecule compound.”
Samumed, now Biosplice Therapeutics, rebranded SM04554 as Dalorsirvat, but most people still refer to it as SM04554.
SM04554 is a novel small molecule Wnt activator that regulates the Wnt pathway.
SM04554 increased the specific sum of nuclear β-catenin, versican, and Ki-67 in hair follicles, indicating local follicle activation of Wnt signaling and proliferation, rather than generalization to the epidermis. SM04554 increased the number of hair follicles and hair growth compared to the carrier.
SM04554 works by turning on the Wnt signaling pathway in human cells and is able to work quickly, initiating and maintaining hair growth, while other hair loss medications take a long time to work and progress more slowly.
It can be said that our mainstream understanding of androgenic alopecia is still stuck at the level of the middle of the last century, and it is always believed that hair loss is closely related to genes and the male hormone DHT.
In fact, Such as Wnt signaling pathway modulators are relatively new research achievements in the new century (WNT signaling pathway modulator SM04554, JAK inhibitor ATI-50002, androgen receptor antagonist clascoterone, CRTH2 antagonist setipiprant, thyroid hormone receptor agonist PF-002773) 43 etc.).
In the body, there are progenitor stem cells that can repair certain organs, and this process of repair is initiated by a group of proteins called the Wnt pathway. If the number of certain Wnt genes is tweaked by a compound, progenitor stem cells can be triggered to repair certain organs.
In simple terms, the Wnt pathway is a group of protein pathways that transmit signals into cells via cell surface receptors. Wnt is an ancient signaling pathway that dates back hundreds of millions of years to evolutionary processes.
The Wnt pathway plays a crucial role in cell growth and migration and is key to embryonic development. It is also involved in wound healing and recovery from injury.
The Wnt signaling pathway is like a regulatory switch for cell regeneration, and the use of compounds to inhibit or activate the Wnt signaling pathway ultimately makes it possible to regenerate tissues and treat certain diseases that were irreversible in the past.
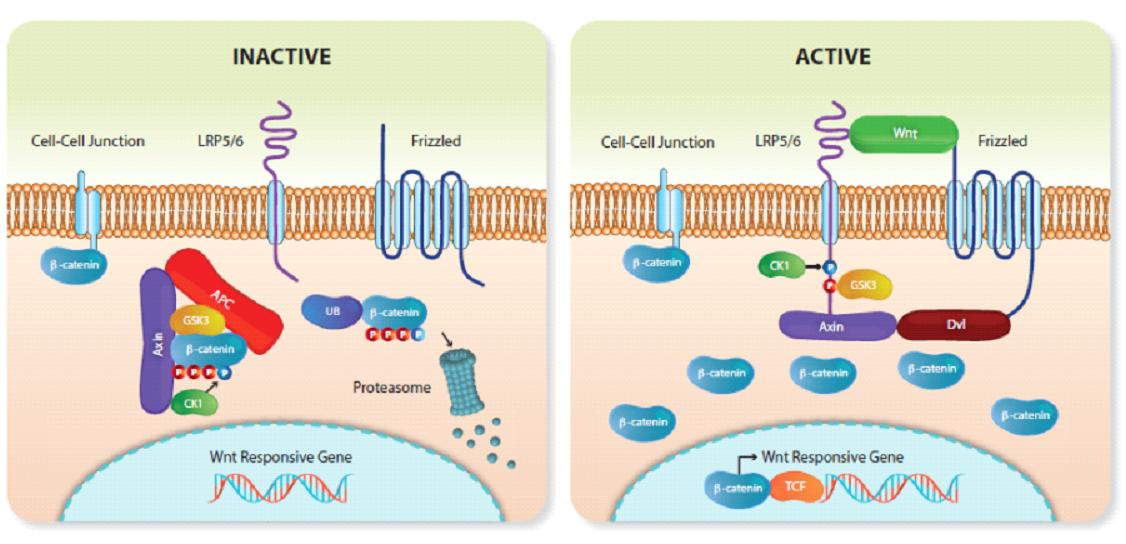
Studies have long demonstrated the relationship between the Wnt signaling pathway and hair growth, and the activity of the Wnt pathway is significantly reduced in AGA patients (male AGA is characterized by progressive hair follicle shrinkage and reduced hair growth).
Therefore, improving the activity of the Wnt pathway can improve hair problems.
And this is the new approach in treatment of androgen alopecia, and on the principle of finasteride, RU58841, CB301 traditional antiandrogen drug has essential difference.
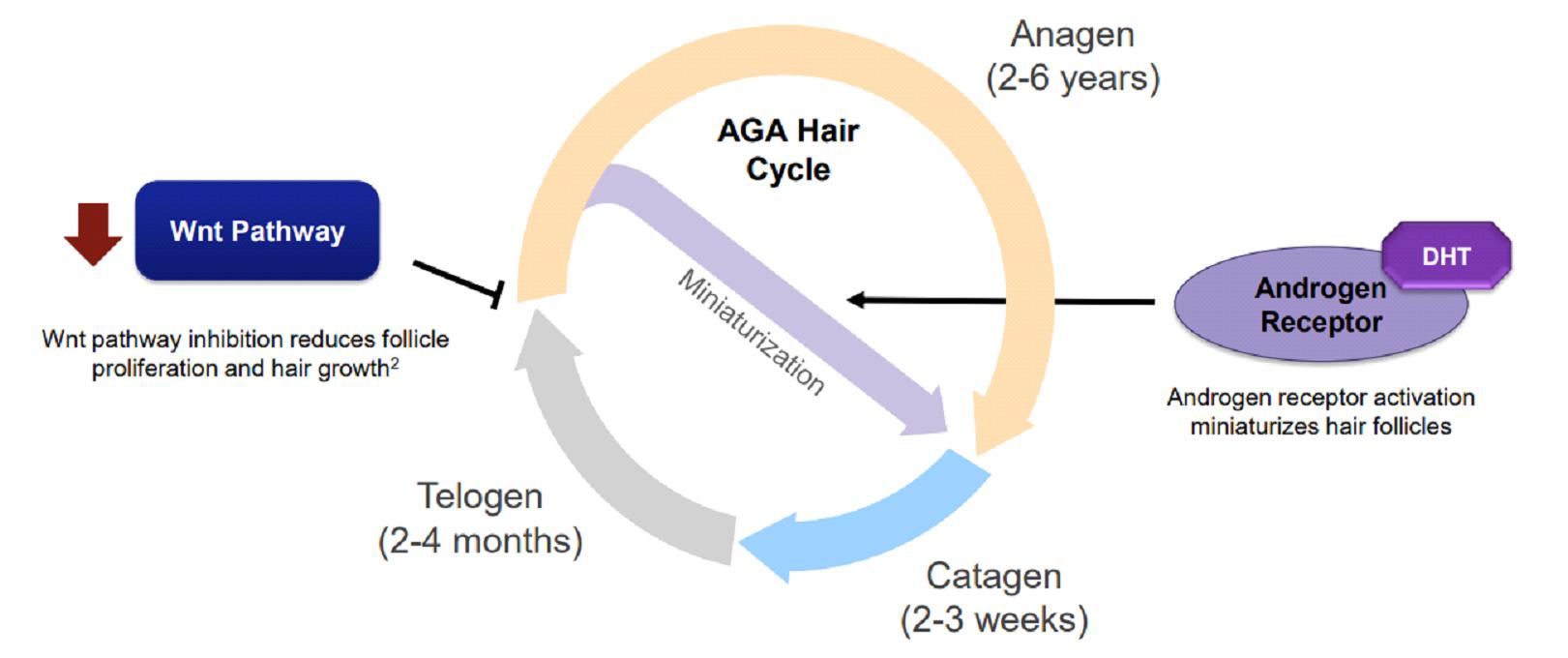
4.SM04554 induces endogenous dermal progenitor cells to differentiate into hair follicles, thus forming new hair follicles.
As male pattern baldness progresses, the hair becomes thinner. Hair follicles become so small that they can no longer grow hair. Eventually, the hair follicles die completely.
SM04554 stimulates hair follicle cells to resume differentiation by regulating the Wnt pathway until hair follicles regenerate, so that damaged hair follicles can be repaired. At the same time, it brings nutrients, oxygen and blood flow to the hair follicles. This will promote healthy hair regrowth.
Therefore, SM04554 is also effective for patients with advanced hair loss and damaged hair follicles.
SM04554 has completed all three phases of inspection.
The Phase I trial of SM04554 involved 29 participants for 28 days and reported no adverse effects other than one adverse event of eye irritation.
The Phase I trial of SM04554 involved 49 participants for 90 days and was followed for 45 days.
0.25% concentration and 0.15% concentration of SM04554 increased hair follicles as shown in the figure below:
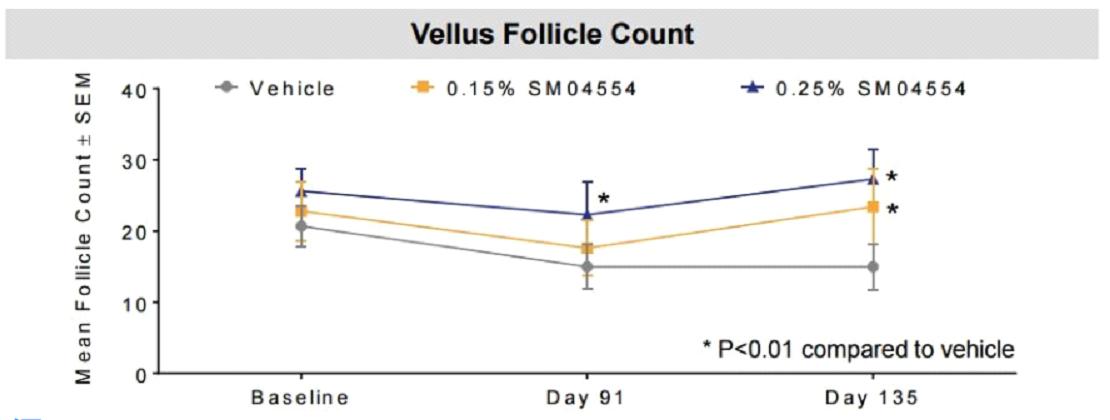
The final hair of 0.25% concentration and 0.15% concentration of SM04554 increased as shown in the figure below:
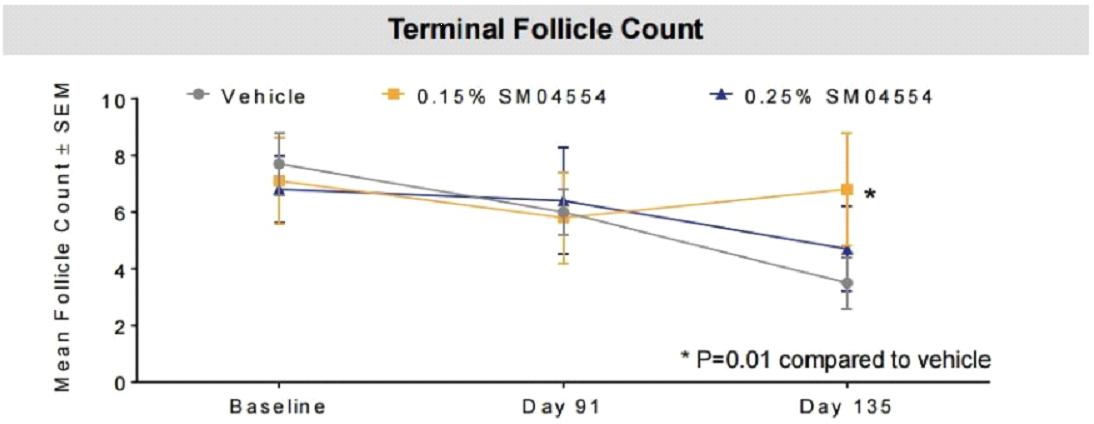
The effects of 0.25% concentration and 0.15% concentration of SM04554 on the total hair follicles in the final growth phase are shown in the figure below:
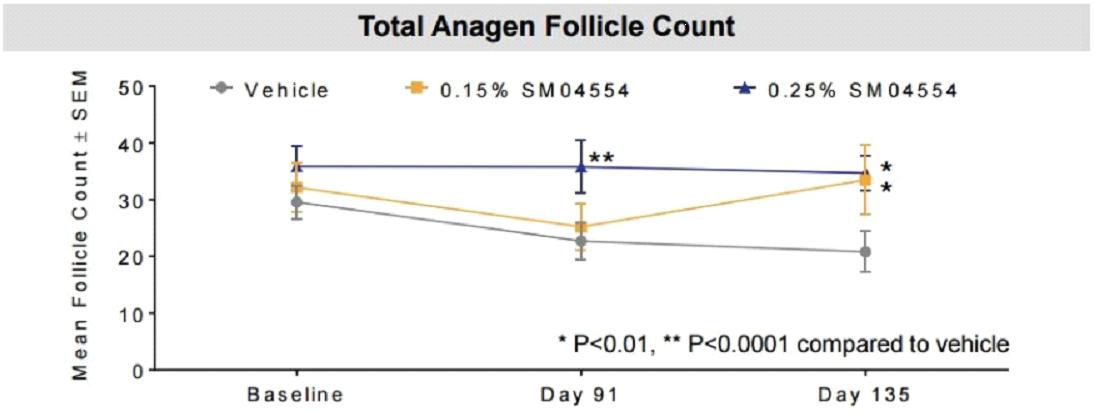
The Phase II trial of SM04554 involved 300 participants and lasted 135 days.
The Phase 3 trial of SM04554, which began in 2018 and was completed in 2021, involved 625 AGA patients, but Biosplice Therapeutics did not release the results of the trial.
In response to an email inquiry from the public, Biosplice sent the following response:
“Biosplice is not pursuing further independent development of SM04554 for androgenetic alopecia and is evaluating global out-licensing opportunities for further development and commercialization of this program.”
This means that Biosplice may not spend any more money and effort researching SM04554, or it may sell the rights to SM04554 to another company.
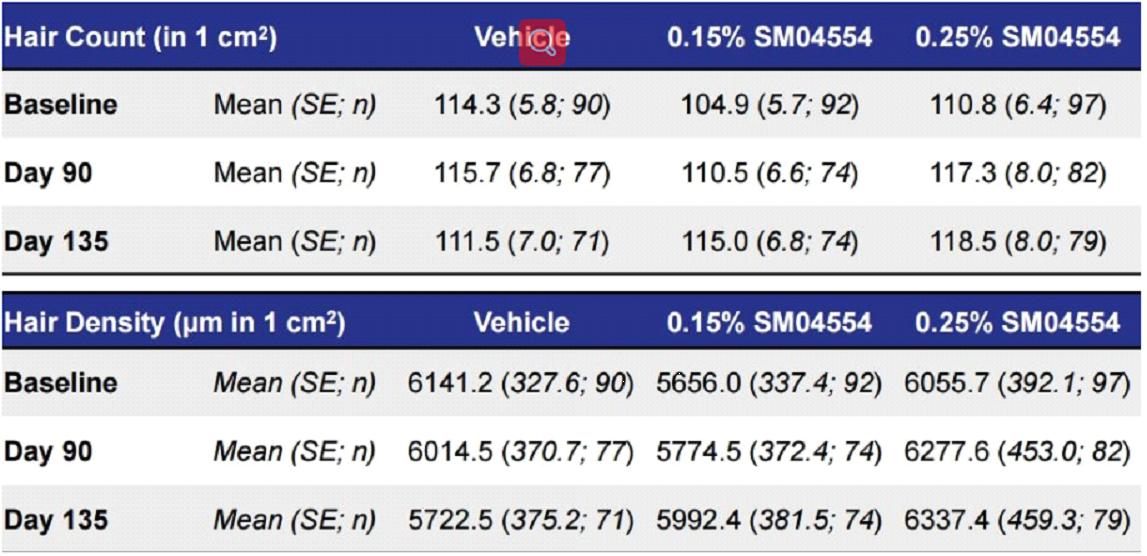
Because the phase III clinical data of SM04554 has not been disclosed, we can understand the product that is still under study from the phase II clinical data of SM04554.
The data from the Phase II clinical trial produced a surprising result: lower doses were more effective.
Phase II clinical trials show that:
Men who used the placebo solution lost an average of 114 hairs /m2 during the study period to 111.5 hairs /m2
Men who used a lower dose of 0.15% SM04554 saw their hair count increase from 104.9 at the start to 115 after treatment
Men using a higher 0.25% dose of SM04554 had an average hair count increase from 110.8 to 118.5.
According to a press release from Biosplice, ‘The findings are consistent with preclinical in vivo animal models, in which SM04554 has been shown to generate new hair follicles and increase hair count’.
From the experimental data, hair continued to fall out despite the use of placebo, and after using SM04554 for three months, hair stopped falling out and the density increased by 10% overall. After stopping treatment, hair continued to grow. Also, side effects are minimal or nonexistent.
At the same time, it is important to note that the patients treated were all high Norwoods(grades 4 to 6) on the Norwood-Hamilton Pattern baldness classification, indicating that SM04554 is effective for advanced hair loss.
This is good news to be happy about.
However, a report in Forbes suggests that the results could have implications for the drug’s future development, given that the lower of the two doses tested during the trial proved to be more effective at hair regeneration than the higher dose.
Because, typically, drugmakers like to see what’s called a dose response: the more drugs you give, the more potency and side effects you get. So, for some companies, a lack of dose response can be seen as a reason to cancel drug development plans.
SM04554 is for topical use, applied directly to the scalp in 0.15% and 0.25% concentrations.
In clinical trials of SM04554, long-term results were better when used in low doses, so a more appropriate dose is 0.15% once daily.
The most common side effects of SM04554 in trials were redness, burning, or tingling at the application site, mostly 1 or 2 on a severity scale of 1-5. None of these side effects stopped a subject from dropping out of the trial, and they all disappeared after a few days.
It is probably because the onset age of female pattern hair loss is mostly after reproductive age, which is later than AGA, and women’s hair is usually longer than men’s, so even if there is a small amount of hair loss, it will be covered by long hair. So, comparative hair loss is not as high a concern as it is for men.
What we commonly call female pattern hair loss, also known as female androgenic alopecia (FPHL), is also caused by androgens.
In healthy women, about 6-38% experience some degree of frontal or frontal parietal hair loss.
The clinical studies on androgen alopecia are mostly for men, and the clinical trials did not enroll patients with female androgen alopecia, including SM04554.
Biosplice is clearly aware of this as well, so Biosplice’s plan is to expand the testing to women, which is clearly needed, FDA-approved finasteride is basically used in male hair loss patients, and female hair loss patients are usually only able to choose minoxidil, If SM04554 sees positive results in trials with women, it will be a big win for Biosplice.
While Biosplice did not conduct the SM04554 trial for female hair loss, in 2016, a trial involving activating the Wnt/ beta-catenin pathway for the treatment of female pattern hair loss confirmed the positive effects of activating the Wnt/ beta-catenin pathway for female pattern hair loss.
The study included 20 patients with female pattern alopecia, aged 25 to 57 years, in an uncontrolled, open-label, six-month study.
Compared to baseline, hair quantity and hair quality index were found to have increased by 6% each at 6 months (P < 0.01) and 12% (P < 0.001). None of the participants stopped treatment due to adverse reactions, and overall patient satisfaction was good. At the molecular level, local application of the study product resulted in a 32% increase in the expression level of WNT10B mRNA in the temporal scalp region (P < 0.001)
The results of this experiment show that topical application of Wnt/ beta-catenin pathway activator can induce the expression of WNT10B in the scalp to increase hair quantity and hair quality index, and indicate that this approach is a new strategy for the treatment of female pattern hair loss.